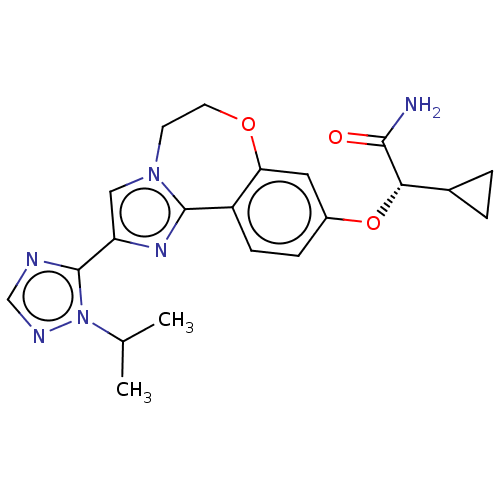
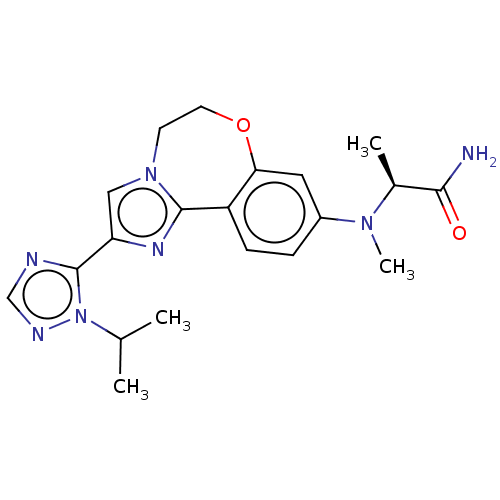
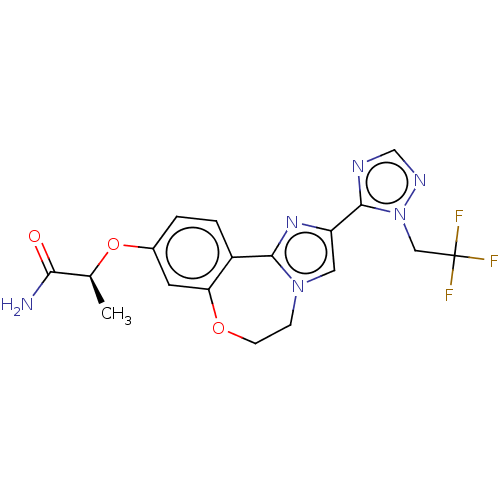
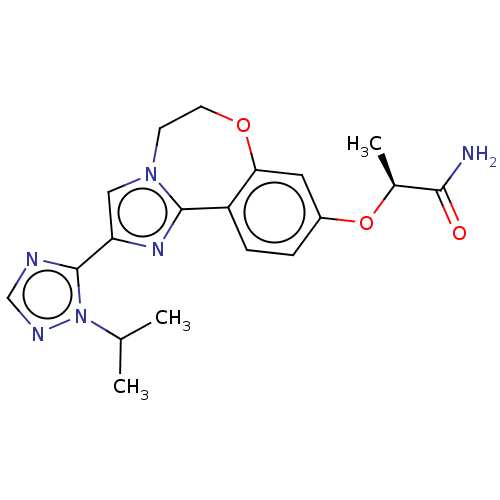
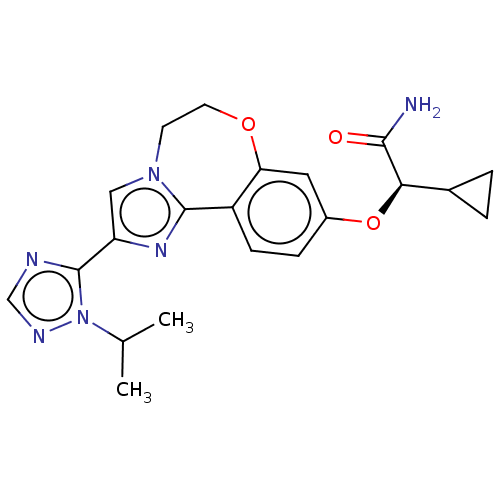
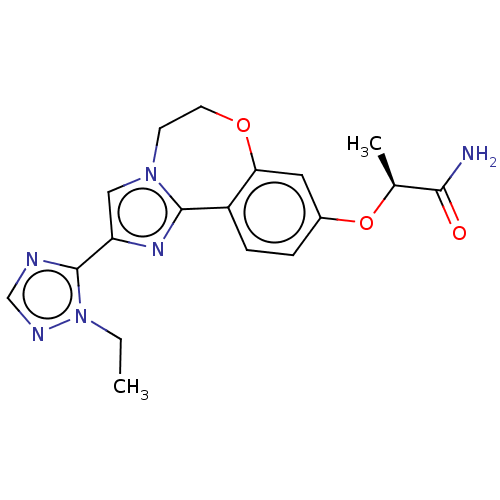
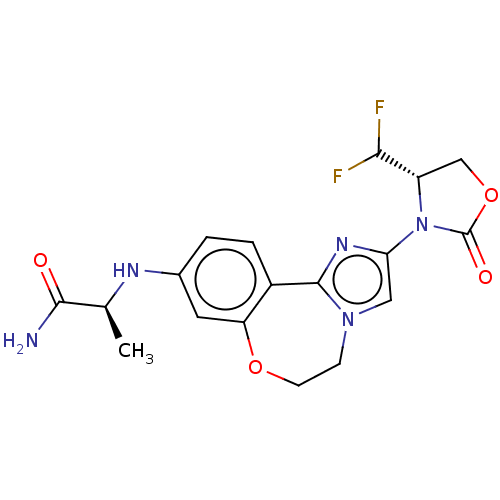
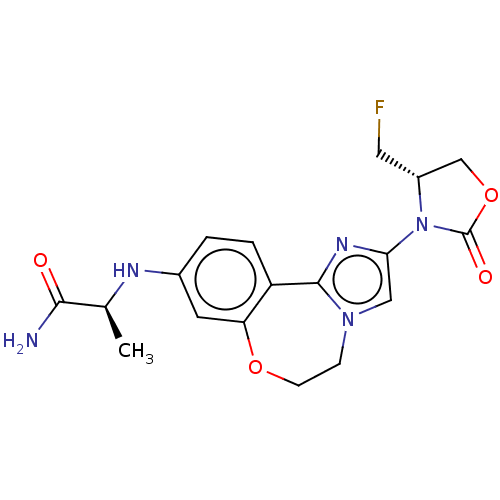
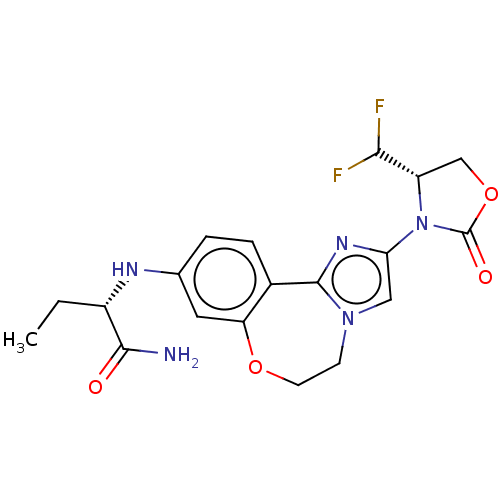
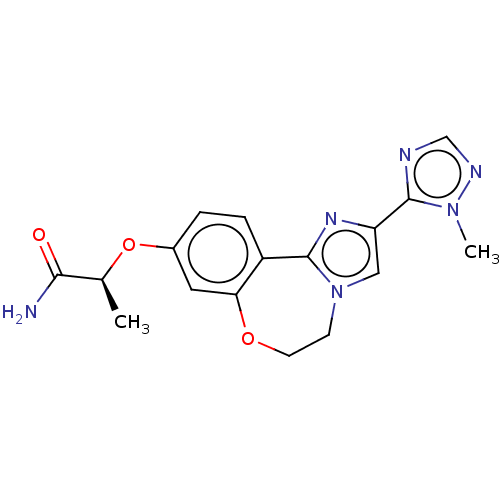
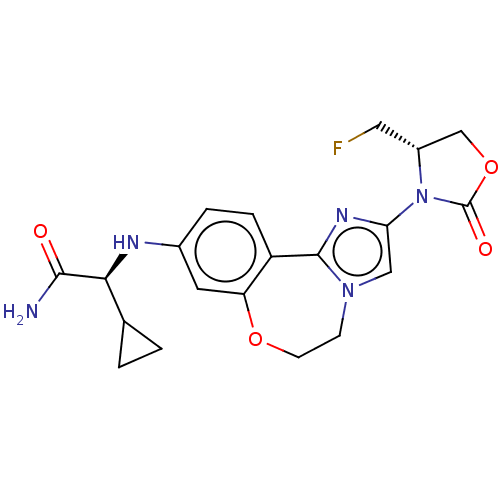
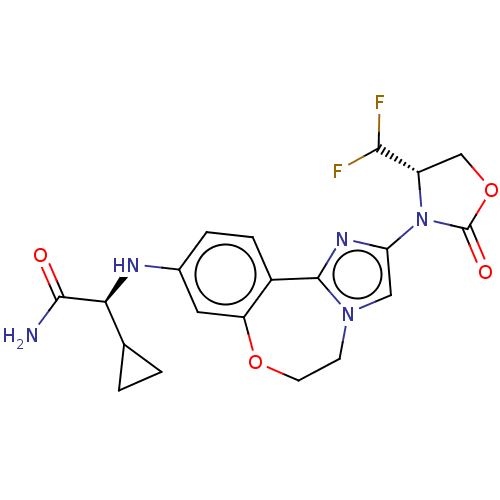
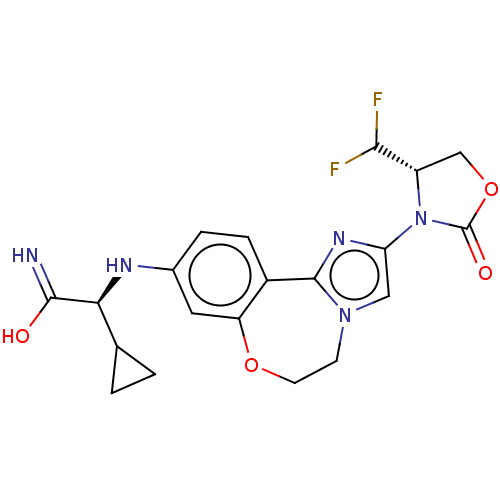
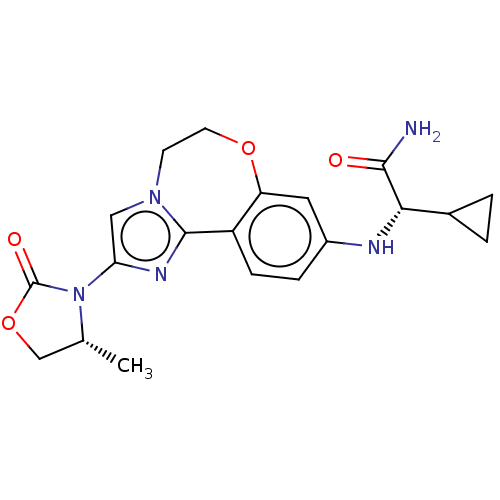
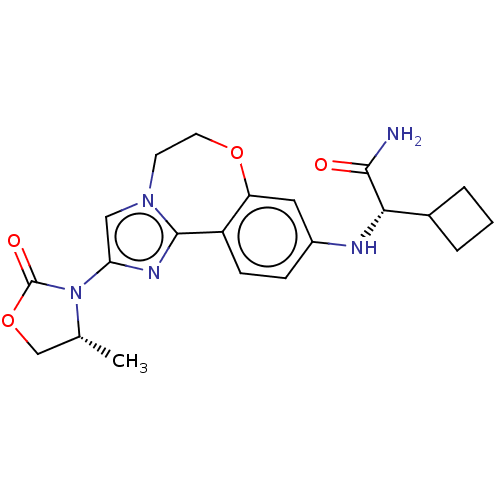
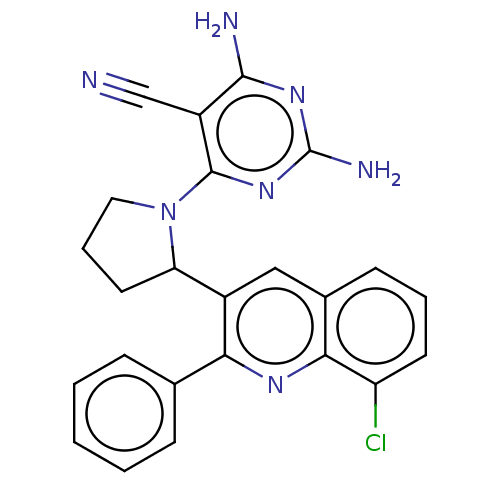
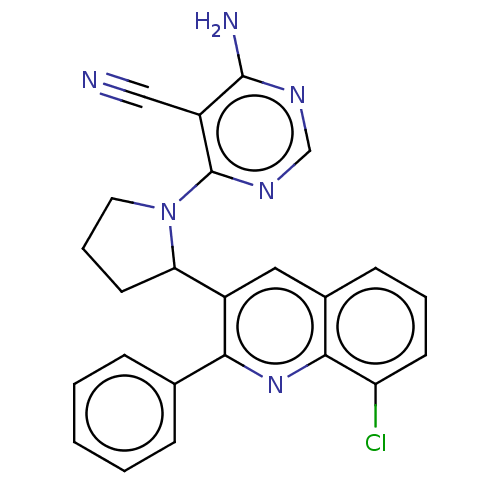
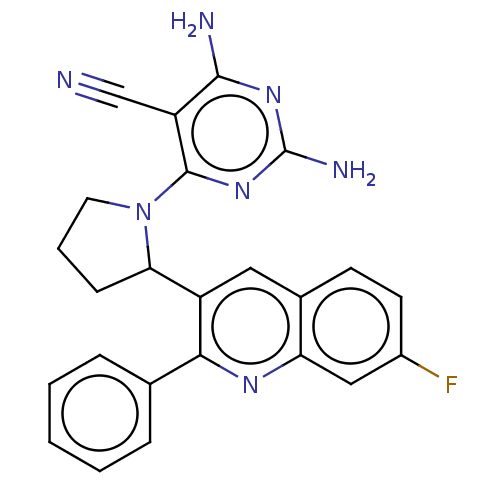
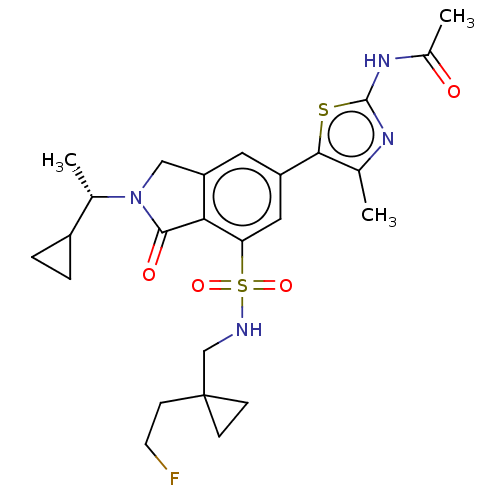
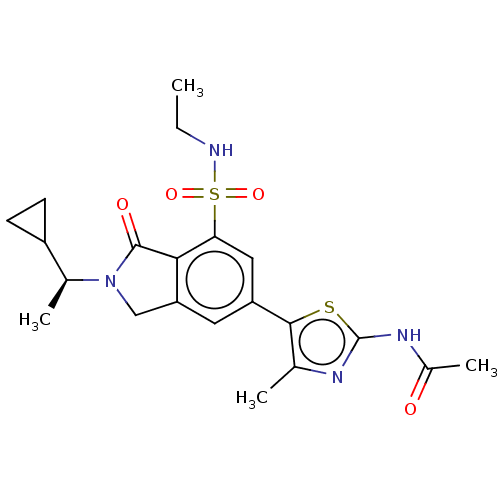
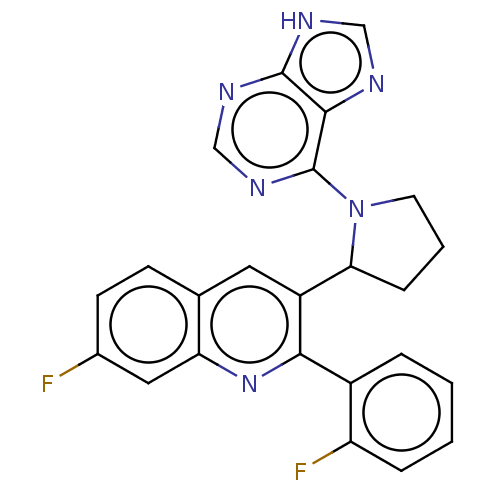
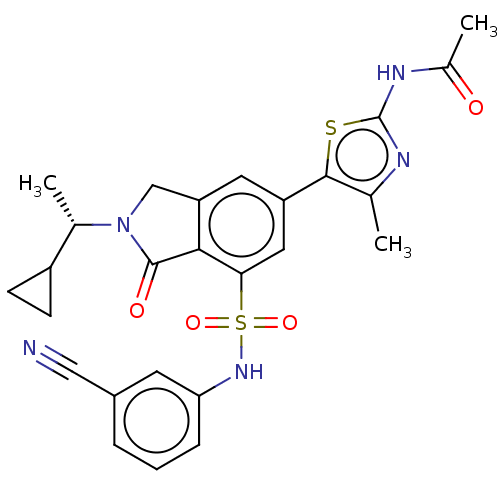
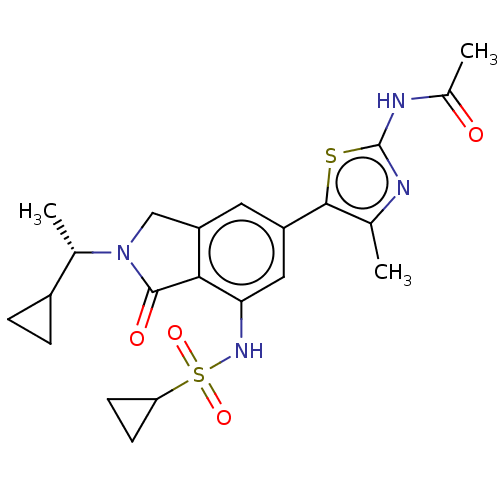
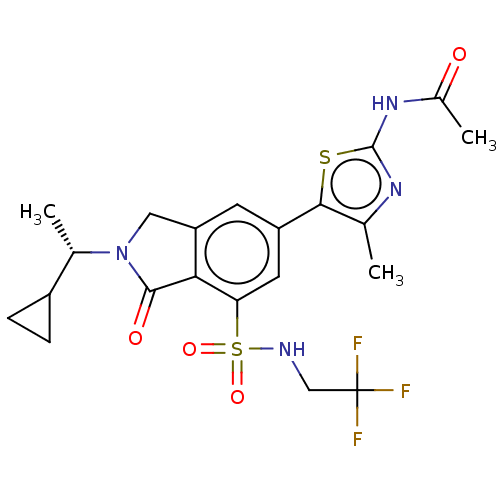
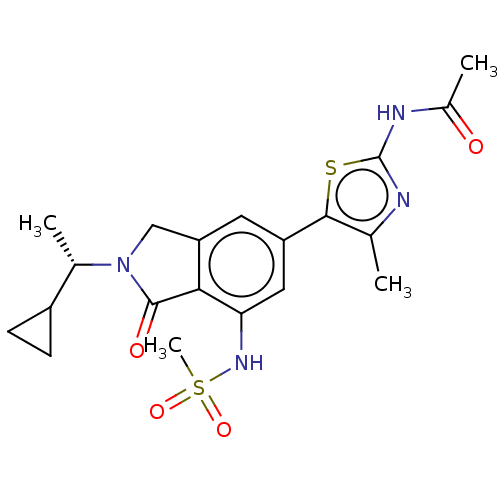
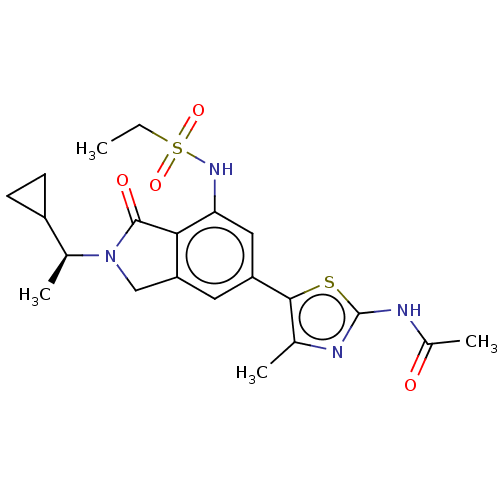
Found 4675 of Enz. Inhib. data with enzyme = 'Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform' and Substrate = 'to-be-curated'
Found 4675 of Enz. Inhib. data with enzyme = 'Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform' and Substrate = 'to-be-curated' TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
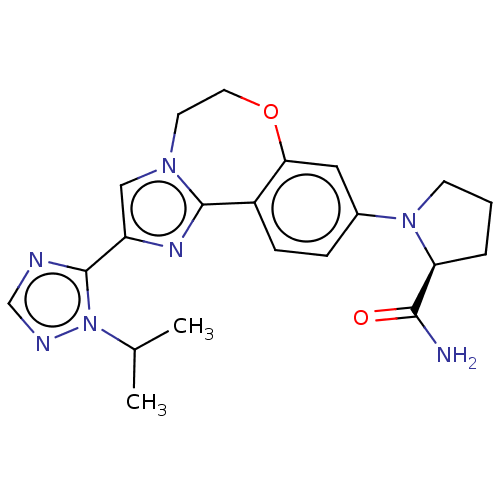
Affinity DataKi: 0.0790nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0790nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
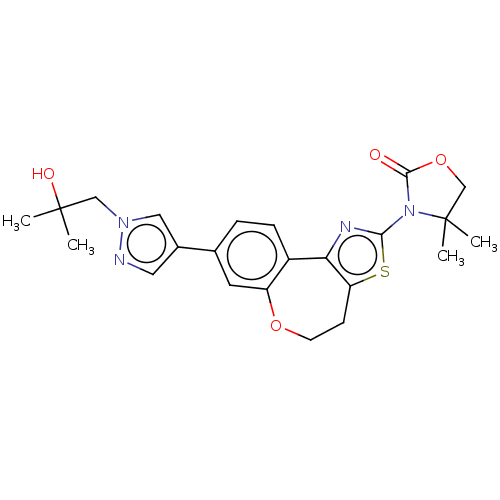
Affinity DataKi: 0.390nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.417nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
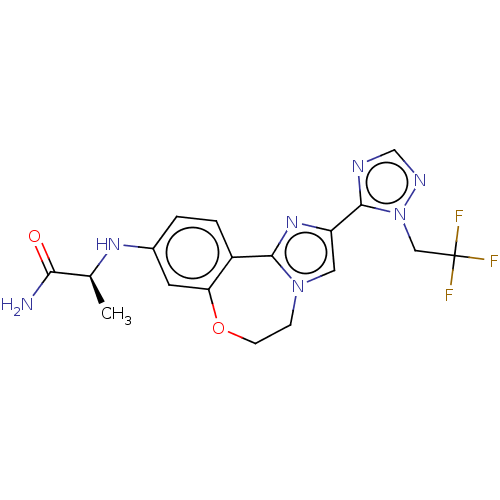
Affinity DataKi: 0.920nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 1.05nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
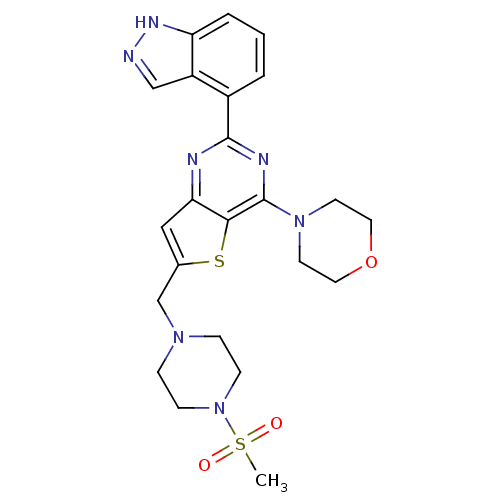
Affinity DataKi: 1.43nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 1.54nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 1.70nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 1.96nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 2.5nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 6.94nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 9.41nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 11.3nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 12.2nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 16.7nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 17.5nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 17.8nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 18.2nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 21.8nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 23.7nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 24.1nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 30.3nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 36.4nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 37.7nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 41.8nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 49nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 197nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 286nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 289nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 708nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: <0.0500nMAssay Description:Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: <0.0500nMAssay Description:Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: <0.0500nMAssay Description:Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: <0.0500nMAssay Description:Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: <0.0500nMAssay Description:Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: <0.0500nMAssay Description:Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.100nMAssay Description:Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.398nMAssay Description:The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.398nMAssay Description:The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.5nMAssay Description:Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.501nMAssay Description:The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.631nMAssay Description:The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.631nMAssay Description:The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.631nMAssay Description:The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.631nMAssay Description:The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.631nMAssay Description:The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.700nMAssay Description:Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.794nMAssay Description:The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataIC50: 0.794nMAssay Description:The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c...More data for this Ligand-Target Pair
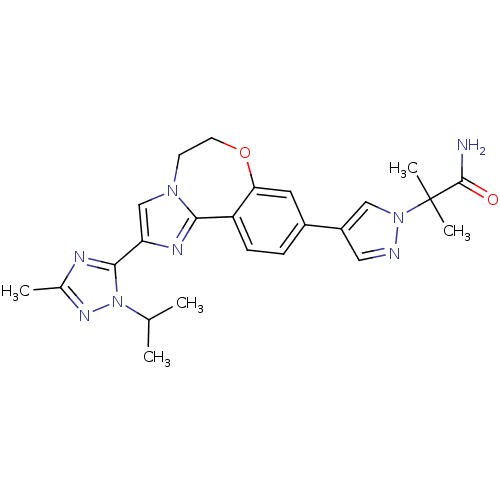
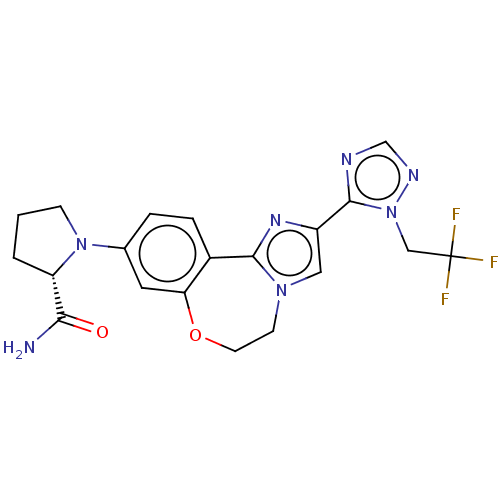
 3D Structure (crystal)
3D Structure (crystal)